
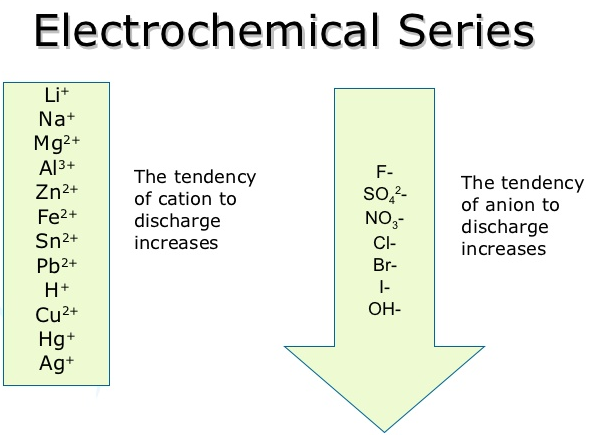
Metal ion reactivity series series#
Understanding the reactivity series is critical in the fight against corrosion. Copper and silver, on the other hand, may be extracted using less reactive procedures such as smelting and reduction. Electrolysis is used to obtain highly reactive metals such as potassium and sodium. The reactivity series is important in metallurgy, which is the science of recovering metals from their ores. Furthermore, by distinguishing the elements undergoing oxidation and reduction, the reactivity series aids in balancing redox equations. Highly reactive components are more likely to react with other substances, whereas less reactive elements are less likely to react. The reactivity series can be used to forecast the results of chemical processes. Significance of the Reactivity Series Predicting and Balancing Reactions Because of their stability, noble metals may resist corrosion and stay in their pristine state in nature.
Metal ion reactivity series full#
They have stable electron configurations with entire or almost full outer electron shells, which makes them less reactive than other compounds. Noble metals such as gold and platinum are distinguished by their low reactivity. Because of their capacity to assist chemical processes without being consumed in the process, transition metals are frequently utilized as catalysts. They may form compounds with a wide spectrum of elements and have numerous oxidation states. Transition metals with moderate reactivity include iron and zinc.
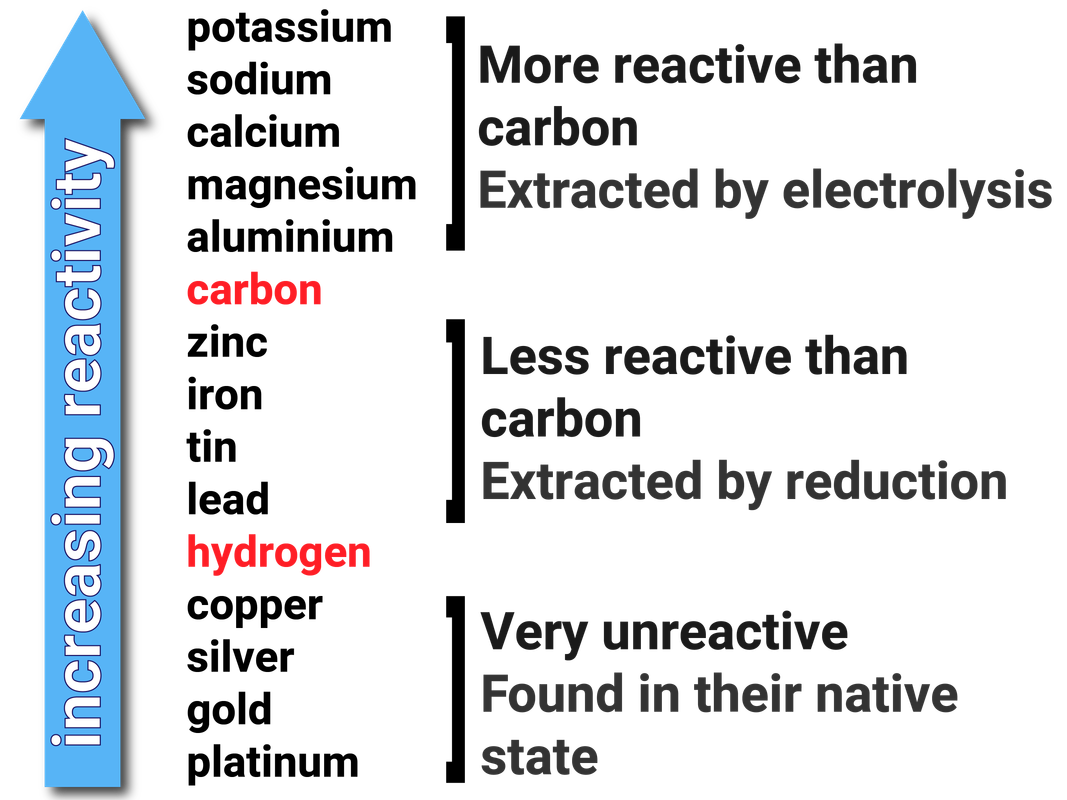
The diminishing strength of the metallic link retaining the outermost electron enhances reactivity as we proceed down the group. Because of their low ionization energies, they easily shed their outermost electrons, generating positive ions. Understanding Reactivity Trends Alkali Metals and Alkaline Earth MetalsĪlkali metals such as potassium, sodium, and calcium are all highly reactive elements. It is important to note that this list provides a general guideline and may vary slightly depending on the specific reaction being considered. Here is a commonly accepted order of elements in the reactivity series: The reactivity series follows a general order, although slight variations can occur based on specific reaction conditions. This concept is essential in understanding various chemical phenomena, such as the extraction of metals, predicting reaction outcomes, and preventing corrosion. Elements at the top of the reactivity series are the most reactive, while those at the bottom are the least reactive. The series allows scientists to predict the behavior of elements when they come into contact with other substances and provides insights into the types of chemical reactions they are likely to undergo. It provides a systematic framework for comparing and ranking elements according to their reactivity.

The reactivity series is a hierarchical arrangement of elements based on their relative tendency to undergo chemical reactions. After reading this article, you will be able to understand the nature of the Reactivity Series as well as its uses and functions. In this article, you will learn about the Reactivity Series, including its significance and its applications.


 0 kommentar(er)
0 kommentar(er)
